India's drug regulator, the CDSCO, conducted quality tests in August that revealed a number of medications for diabetes, hypertension, vitamins, calcium D3 supplements, bacterial infections in children, acid reflux, and stomach infections did not meet quality standards.
The Central Drugs Standard Control Organization (CDSCO) of India released a comprehensive report in August 2024. This report listed several vital drugs commonly used across the country as failing the quality test. These included medications for diabetes, hypertension, acid reflux, vitamins, calcium supplements, and antibiotics for treating bacterial infections in children. According to the report, these medications were declared NSQ (Not of Standard Quality).
Source: aajtak
Drugs That Failed the Test:
The report revealed that these drugs did not meet quality standards.
Paracetamol Tablets (500 mg):
Often used for mild fever and as a pain reliever, it's a common fixture in households.
Glimiperide:
This anti-diabetic drug, manufactured by Alkem Health, is used for managing sugar levels.
Telma H (Telmisartan 40 mg):
This Glenmark medication for high blood pressure also failed to meet standards.
Pan D:
Typically used for treating acid reflux, this Alkem Health Science drug did not pass the quality test.
Shelcal C and D3 Calcium Supplements:
Manufactured by Pure & Cure Healthcare and distributed by Torrent Pharmaceuticals, Shelcal failed to meet the standards.
Clavam 625:
An antibiotic drug that also failed the test.
Cepodem XP 50 Dry Suspension:
Used for treating serious bacterial infections in children, this drug by Hyderabad's Hetero Company did not pass the quality test.
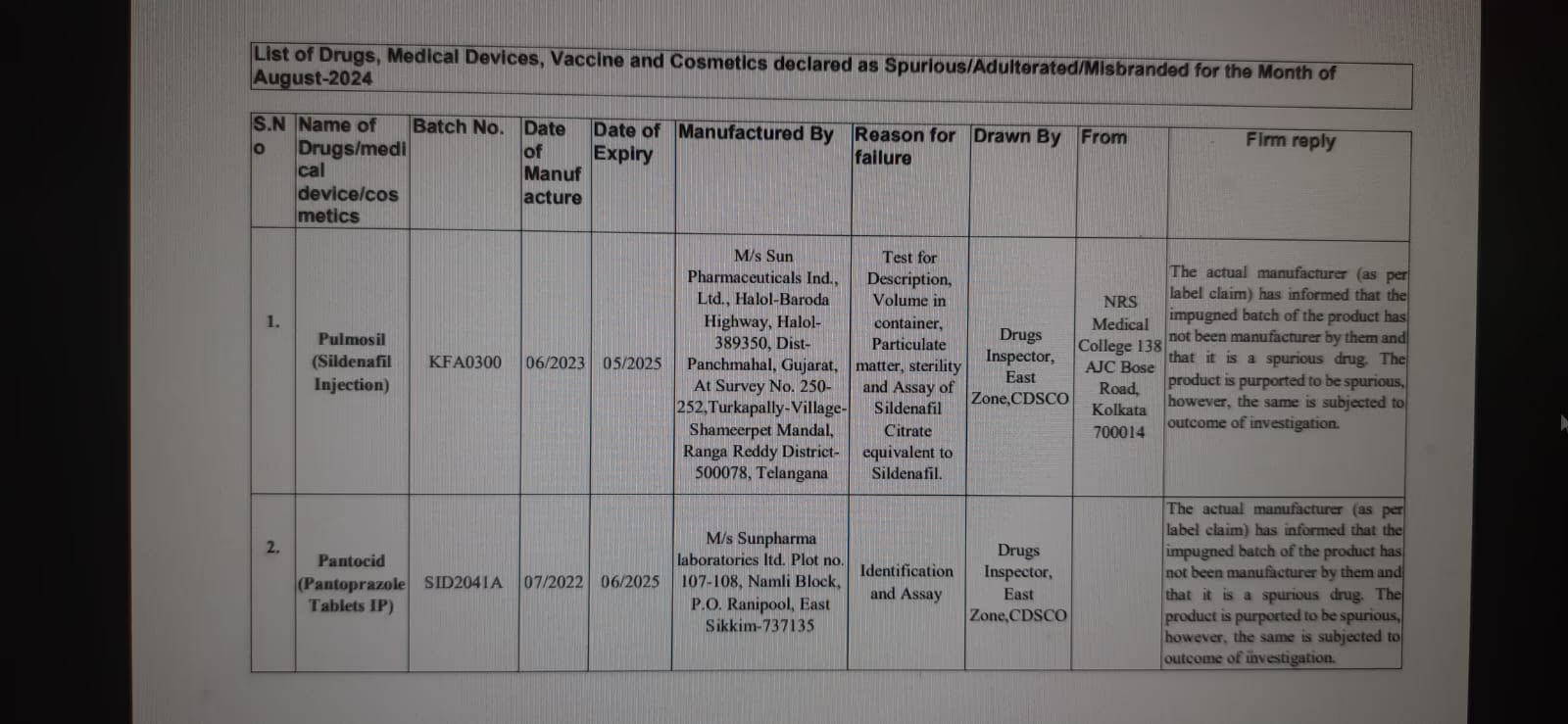
Pulmosil (for Erectile Dysfunction):
Manufactured by Sun Pharma, this drug used to treat erectile dysfunction also failed to meet quality standards.
Pantocid (for Acid Reflux):
Another Sun Pharma drug for acidity and reflux, which did not pass the test.
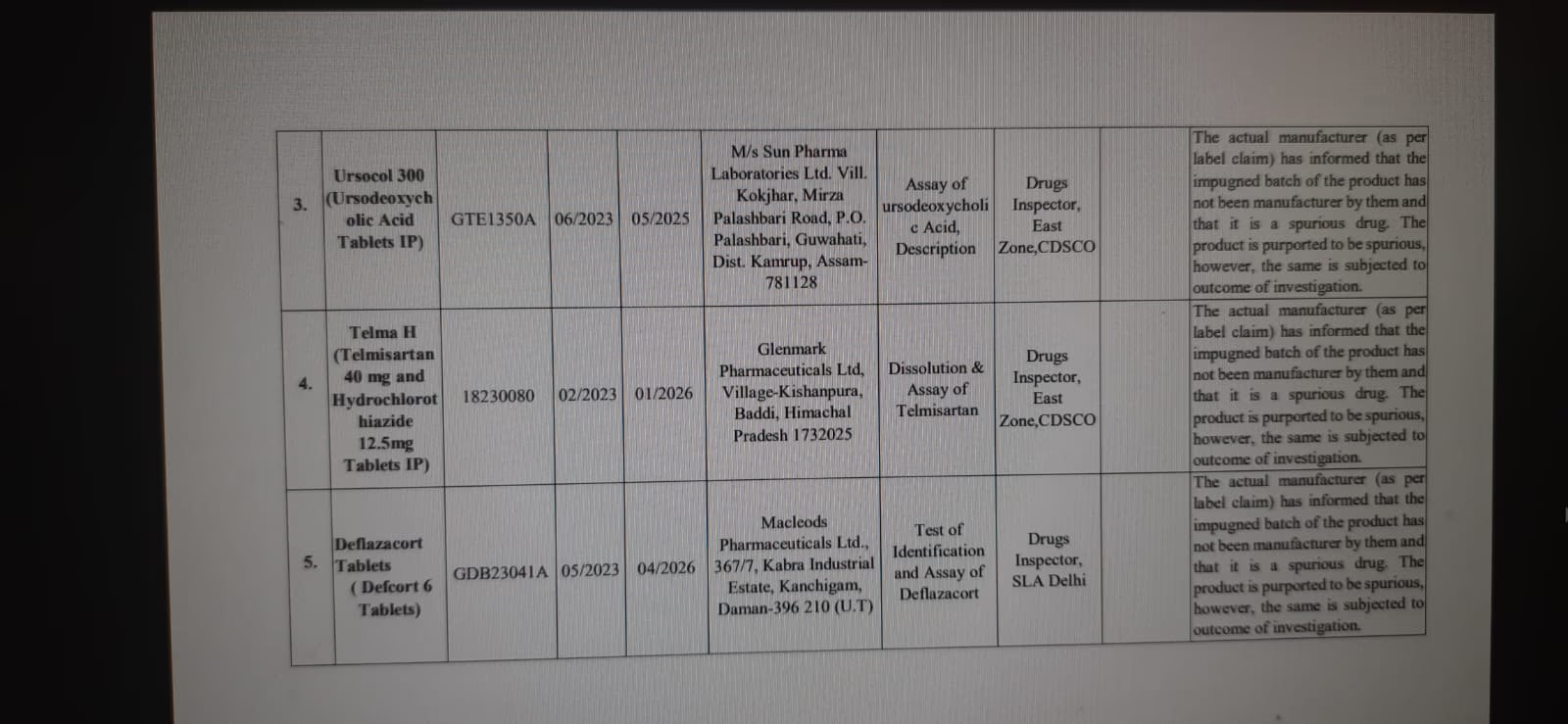
Ursocol 300:
This drug by Sun Pharma also failed to match the quality standards.
Defcort 6:
Used for treating arthritis, this Macleods Pharma drug did not meet the quality standards.
Source: aajtak
Company Responses:
After the test reports surfaced, the respective companies responded by claiming that the batches mentioned in the report were not produced by them and may be counterfeit. They also stated that they are awaiting the results of the investigation.
CDSCO's Statement:
CDSCO stated that the report depended on the investigation results concerning the production of counterfeit drugs. For now, the regulatory agency is looking into whether these drugs were introduced to the market as counterfeits or if they were manufactured in violation of standards. Until the investigation results are out, these drugs are not banned from the market. However, the regulatory body has asked the concerned companies to take necessary measures.
Potential Risks:
Drugs failing quality standards can pose severe health risks to patients. If counterfeit drugs are entering the market, it not only affects medical treatments but also raises significant concerns about the country's healthcare system. The investigation by CDSCO highlights the gravity of this issue and underscores the need for stringent monitoring in the pharmaceutical industry.
What is CDSCO?
The Central Drugs Standard Control Organization (CDSCO) is India's national regulatory body for drugs, medical devices, and cosmetics. It functions under the Ministry of Health and Family Welfare. CDSCO acts as a regulatory authority, ensuring drugs, medical devices, and cosmetics available in the country are safe, effective, and meet quality standards.
Roles of CDSCO:
Licensing and Registration of Drugs:
Issuing licenses for the market introduction of new drugs and medical devices.
Monitoring Quality Standards:
Ensuring drugs and medical devices conform to safety and quality standards.
Approval for Clinical Trials:
Permitting trials to evaluate the safety and efficacy of drugs and devices.
Drug Quality Control:
Supervising the production and sale of drugs and taking actions against products not meeting quality standards.
Regular Inspections:
Conducting nationwide inspections of drug manufacturing units to review quality standards.
Pharmacovigilance:
Ensuring the process of monitoring and reporting adverse effects during drug use.