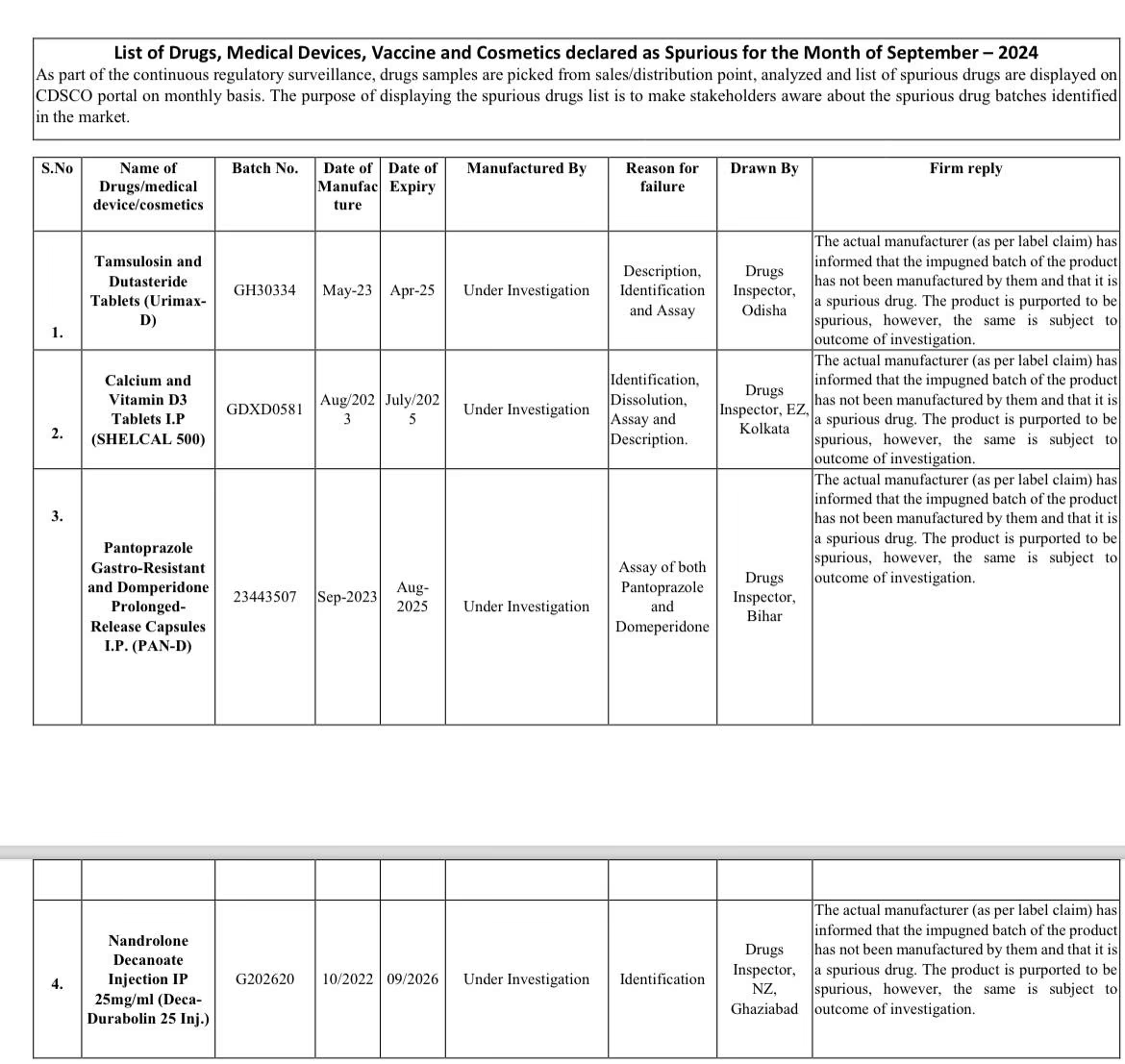
The Central Drugs Standard Control Organization (CDSCO) has released its monthly list for September, highlighting medicines that failed to meet quality standards. In an announcement made on Thursday, CDSCO declared specific batches of four drugs, including the calcium supplement Shelcal 500 and the antacid Pan D, as counterfeit. Furthermore, 49 drugs and formulations were deemed below standard quality.
According to a recent report, the CDSCO's monthly update for September also features Urimax D, used for treating Benign Prostatic Hyperplasia (BPH) or enlargement of the prostate gland, as well as Deca-Durabolin 25 injection, utilized for treating osteoporosis in post-menopausal women. As the manufacturers of these drugs are still under investigation, the alert does not name them, unlike previous months.
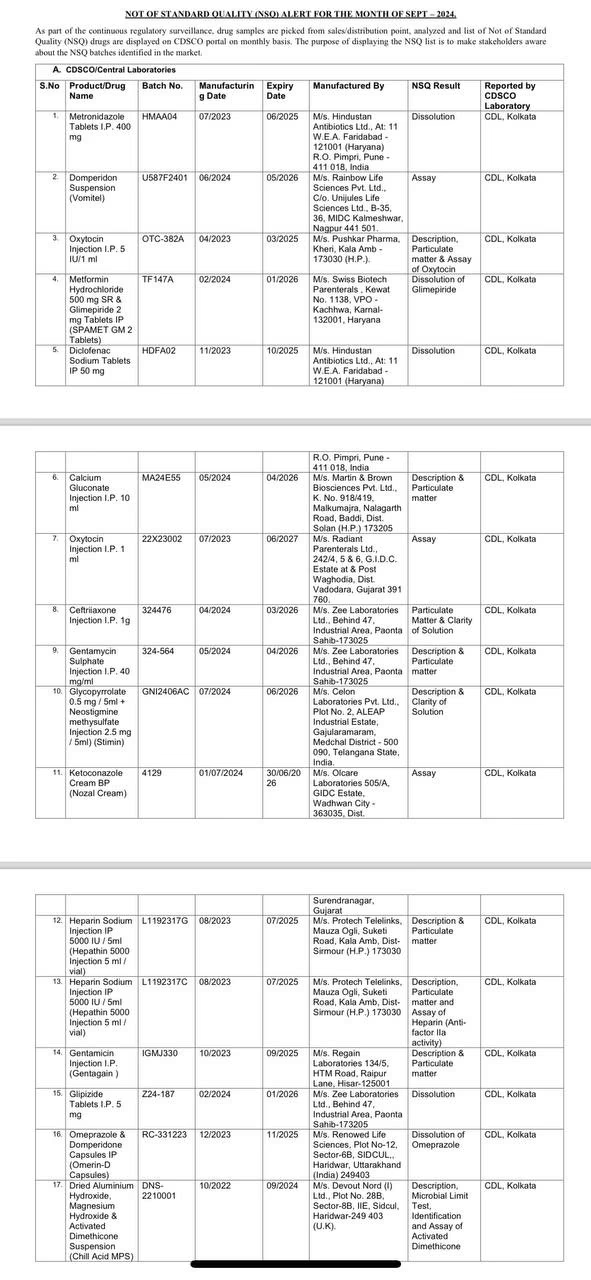
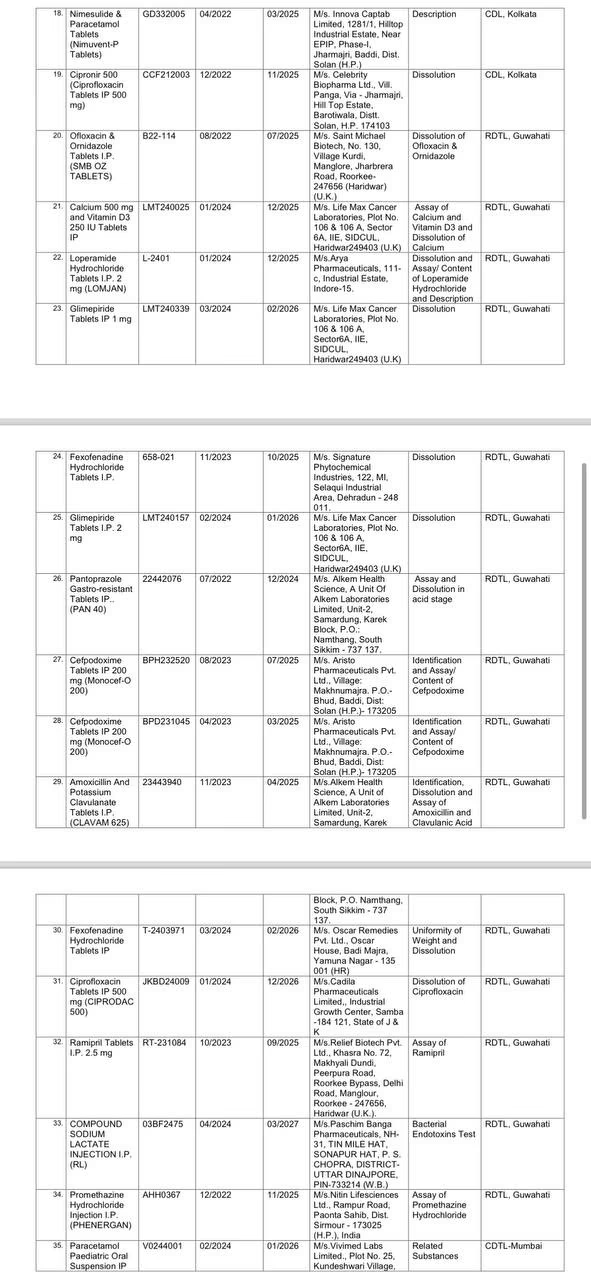
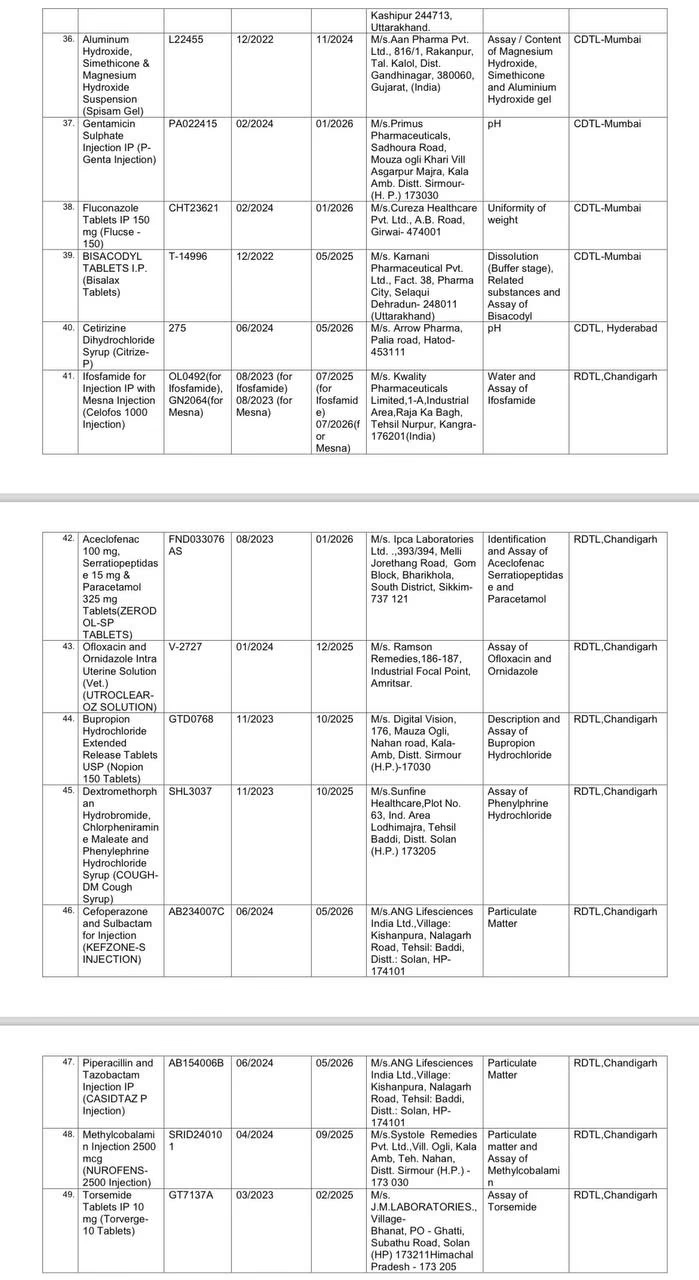
Drugs that Failed the Test:
Source: aajtak
Source: aajtak
Source: aajtak
It should be noted that the CDSCO identified 4 samples produced by counterfeit companies and found them to be counterfeit drugs. Out of 3,000 drugs, 49 failed the quality tests and were withdrawn by CDSCO according to their batches. This vigilant monthly action by CDSCO reduces the percentage of non-standard quality drugs to 1%.
Drug Samples Found Counterfeit:
Source: aajtak
The CDSCO chief stated that only about 1.5% of all the drug samples tested were found to be less effective. This includes Metronidazole tablets by Hindustan Antibiotics, Domperidone tablets by Rainbow Life Sciences, Oxytocin by Pushkar Pharma, Metformin by Swiss Biotech Parenterals, Shelcal 500 mg, and Vitamin D3 250 IU tablets by Life Max Cancer Laboratories, as well as PAN 40 by Alkem Labs.




